The lifetime of NOx varies from ≈6 hours in the boundary layer to several days to a week in the upper troposphere. This leads to a large variation in its concentration: 10 ppbv (parts per billion by volume) in urban areas to 10 pptv (parts per trillion by volume) in remote regions such as the upper troposphere and remote marine boundary layers.
The following table summarizes the principal interchanges between NO, NO2 and HNO3 that are relevant for the chemistry of the troposphere.
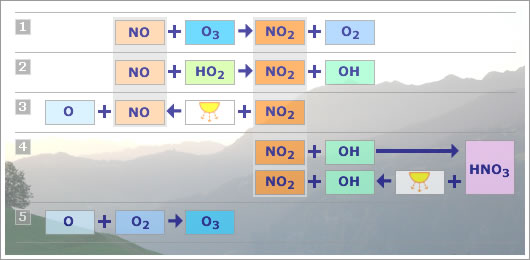
Fig 1.1.3.1: NOx reactions overview.
Image: AT2-ELS
- NO reacts with O3 to create NO2 and O2.
- NO reacts with HO2, the peroxy radical, to create NO2.
- NO2 can be photolysed by sunlight to create NO and an oxygen atom.
- NO2 reacts with OH, the hydroxyl radical, to create HNO3. HNO3 can be photolysed by sunlight to create NO2 and OH once more. HNO3 can thus act as a short term sink for NO2.
- The oxygen atom released in equation 3 can combine with O2 to produce O3; hence the characterisation of NOx as a precursor of ozone.
These reactions are discussed more fully in their context in later sections of this module. For the moment we only need to note the presence of photolytic processes in these relationships, which means that atmospheric NOx will vary between night and day.