NOx plays an important role in the oxidation of hydrocarbons in the lower atmosphere. We shall take the case of the oxidation of methane, CH4, as an example of this process. Methane is a major trace gas in the troposphere, with an average mixing ratio of approximately 1500 ppb.
CH4 reacts with an hydroxyl radical, removing hydrogen to form a methyl radical and water.
| CH4 + OH | → | CH3 + H2O | (1) |
The methyl radical reacts with O2 to form a methylperoxy radical.
| CH3 + O2 | → | CH3O2 | (2) |
In a continental atmosphere during the day, the methylperoxy radical will probably react with the nitric oxide released by combustion processes to form a methoxy radical, CH3O.
| CH3O2 + NO | → | CH3O + NO2 | (3) |
The methoxy radical in turn reacts with O2 to form formaldehyde, HCHO and a hydroperoxy radical, HO2. HO2 produced in this way can also be a significant factor in a catalytic cycle that produces O3 from the photolysis of NO2 (see section 1.1.3.3, 'O3 creation by NO2').
| CH3O + O2 | → | HCHO + HO2 | (4) |
Note also that the formaldehyde molecule itself may be oxidised by OH in a similar sequence of reactions to the oxidation of methane, the end result being the formation of CO or CO2 and H2O).
The hydroperoxy radical from reaction (4) oxidises NO to NO2 and regenerates an OH radical.
| HO2 + NO | → | OH + NO2 | (5) |
Note the following features of this process:
- The reaction sequence (1) to (5) constitutes a chemical chain reaction with OH as the chain carrier: OH is consumed in reaction (1) and regenerated in reaction (5). The OH radical can thus be regarded as a catalyst, only a small amount being needed to oxidise much larger amounts of CH4.
- In the same reaction chain, two molecules of NO are also oxidised to NO2, reactions (3) and (5).
- Volatile organic compounds (VOCs) such as hydrocarbons are all oxidised by similar mechanisms.
The following diagram summarizes the cycle:
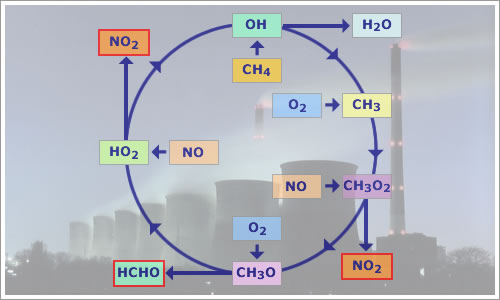
Fig 1.1.3.1.1: Block diagram of the catalytic chain
reaction that oxidises methane, CH4.
Outputs are NO2 and HCHO, and inputs are CH4
and NO.
Image: AT2-ELS