In the presence of relatively high NOx levels and if hydrogen peroxy radicals (HO2) or hydrocarbons are present then additional tropospheric O3 is produced.
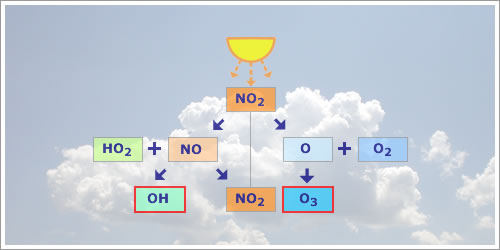
Fig 1.1.3.3.1: Block diagram of the catalytic cycle that creates O3.
Image: AT2-ELS
As with NOx cycling, O3 is produced in a two-step reaction from the photolysis caused by sunlight:
| NO2 + hν | → | NO + O (λ < 424 nm) | (1) |
| O + O2 | → | O3 | (2) |
As with NOx cycling we saw that NO reacted with O3 to produce NO2 and O2. However, NO can also react with organic peroxy radicals (RO2) or peroxy radicals (HO2):
| RO2 (or HO2) + NO | → | RO (or OH) + NO2 | (4) |
That means that there are two paths by which NO can be oxidized to NO2: by reaction with O3 or reaction with RO2 (HO2).
- The reaction of NO with O3 occurs when no hydrocarbons or CO (or only small amounts of them) are available. As a result of this, little or no NO is observable.
- In contrast, the reaction of NO with RO2 (HO2) occurs when there is a greater concentration of hydrocarbons in the atmosphere. For example, hydrocarbons containing five carbon atoms can produce up to five RO2 (HO2) radicals.
The second reaction can lead to high concentrations of O3 and a smog situation, but it is clear from the processes outlined here that NOx needs to be present for this reaction to take place. NO2 is therefore a factor in air pollution.
It is interesting to note that while the hydrocarbons are consumed in the production of ozone, NOx merely serves as a catalyst.