HNO3 plays an important role in the chemistry of NOx in the atmosphere. Three principal pathways exist for the production of HNO3 from NO2:
1. Hydroxyl radical attack
During the day, when OH is more abundant, nitric acid is produced. NO2 is attacked by the hydroxyl radical, leading to an important loss of NO2:
| NO2 + OH | → | HNO3 | (5) |
However, during the day HNO3 is photolysed very quickly to NO2 and OH.
2. Dinitrogen pentoxide intermediary
At night HNO3 is produced via the combination product dinitrogen pentoxide, N2O5, in a heterogenous reaction (reaction 8, below) which can take place, for example, on fog droplets:
| NO2 + O3 | → | NO3 + O2 | (6) |
| NO2 + NO3 | → | N2O5 | (7) |
| N2O5 + H2O | → | 2 HNO3 | (8) |
3. Absorption of NO2 in water
A further mechanism for the production of HNO3 acid from NO2 is the direct absorption of NO2 in atmospheric water. However, owing to the low solubility of NO2 in water this process produces only small amounts of HNO3:
| 2 NO2(gas) + 2 H2O(liquid) | → | HNO2(aq) + HNO3(aq) | (9) |
However, the absorption of NO2 in a water droplet constitutes a real sink for NOx when the droplet is lost through precipitation.
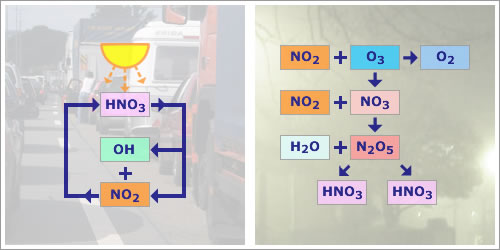
Fig 1.1.3.4.1: Block diagram of HNO3 daytime and night-time reactions.
Image: AT2-ELS
The HNO3 from these reactions should be viewed as a reservoir rather than a terminal product, since it can be the source of radical (or NOx) regeneration. An example of this is the rapid daytime photolysis of HNO3 to NO2 and OH, followed by the rapid reaction of OH with CH4 or other hydrocarbons. HNO3 therefore acts as a sink for NO2 more during the night than during the day.
Apart from its role as a reservoir for NOx, HNO3 precipitation itself is a pollutant which together with sulphuric acid is responsible for the phenomenon of acid rain. Every year acid rain causes damage to a large number of rivers and lakes. In the plant world, damage caused by acid rain can be seen in leaves and fruit. In humans, acid rain can cause respiratory problems. Acid rain also damages buildings, sculptures and monuments.